The Determination of DEA (Diethanolamine) in Refinery Boiler Water Systems Using the IONUS Ion Chromatograph
Alkanolamines, such as Diethanolamine (DEA), are widely used in oil and gas refining and various other industries because of their versatile chemical properties. DEA is an organic compound that contains both an alcohol group and an amine group attached to a backbone of two carbon atoms. These versatile amine products are used in a variety of applications, including the production of consumer goods and critical industrial processes. In particular, the oil and gas industry depend heavily on alkanolamines for acid gas removal in refining and natural gas processing.
Boiler systems are critical components in steam generation for industrial processes, especially within the oil and gas refining industry. The efficient operation of boiler steam production is important for plant operations and minimizing downtime. A key aspect of boiler maintenance is water chemistry management, which includes ensuring proper levels of DEA in the boiler water for the prevention of scaling and corrosion.
Diethanolamine (DEA) is a common alkanolamine for boiler water treatment chemistries to prevent scale and corrosion in the boiler and pipes. Accurate and reliable determination of DEA concentration in the boiler water chemistry is essential for optimizing and maintaining boiler water treatment.
Traditional methods for DEA analysis often report limitations in terms of sensitivity, selectivity, and time efficiency. The literature states that DEA is not easily separated from ammonium or other amines and is difficult to quantify by IC with Conductivity Detection. Although that may be true for other IC systems, the IONUS both separates and detects DEA, making it quite useful for the analysis of DEA and breakdown products for boiler water testing.
The continuous monitoring of amine concentrations in these systems is recommended for maintaining the operational efficiency of the boiler water chemistry. By tracking amine concentrations in the water, process engineers can effectively manage amine makeup, enhance product quality, and reduce system downtime due to scaling or corrosion. While the analysis of alkanolamines is well-established in industrial settings, their determination in boiler waters presents a unique analytical challenge.
The analysis of DEA and any breakdown product produced in the boiler system is essential to understanding the role of DEA in boiler water chemistry, particularly its contribution to pH regulation, oxygen scavenging, and the prevention of scale and corrosion. By accurately quantifying DEA in boiler water samples, it is possible to optimize boiler water treatment programs, improve boiler efficiency, and extend the lifespan of boiler equipment.
By providing a reliable and efficient analytical tool, this application contributes to improved boiler water management, reduced maintenance costs, and enhanced overall refinery operations.
Understanding the Importance of Monitoring DEA in Boiler System
A boiler is a critical component in thermal energy systems, generating steam, and is responsible for converting liquid water into steam through the application of heat energy. Optimal boiler function is contingent upon several factors, including the quality of the water circulating within the system. This water is labeled as boiler water, and is a chemistry of water and amine that influences boiler efficiency, lifespan, and overall operational reliability.
The Role of DEA
To mitigate boiler system degradation, chemical additives are frequently introduced into the boiler water. Diethanolamine (DEA) is one such compound employed to inhibit the formation of scale and mitigate corrosion within the boiler system. It helps prevent two major problems: scale buildup and corrosion.
- Scale: Imagine tiny hard deposits building up inside the boiler. This “scale” can slowly clog the pipes in the steam generation system. Scale reduces the boiler’s efficiency, wastes energy, and can even lead to breakdowns.
- Corrosion: Corrosion is rust eating away at the metal inside the boiler. It weakens the boiler and can cause leaks.
DEA inhibits scale and corrosion formation by regulating the chemical equilibria within the boiler water.
The Importance of Monitoring DEA
To ensure the DEA is doing its job effectively, it’s essential to regularly test the boiler water to confirm the concentration of the DEA and any of its breakdown products. This is where the IONUS Ion Chromatography (IC) comes in. Think of the IONUS IC as a detective, searching for clues about the water’s condition. By analyzing the boiler water with IC, engineers can make accurate DEA determinations and prevent issues with their boiler systems:
- DEA levels: Is there enough DEA in the water to protect the boiler and what is the level of DEA in the boiler?
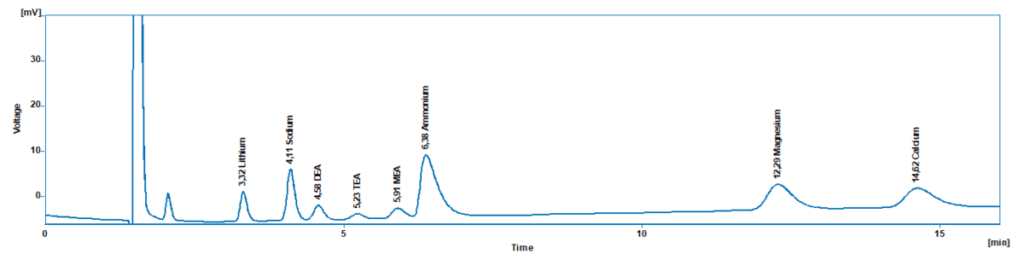
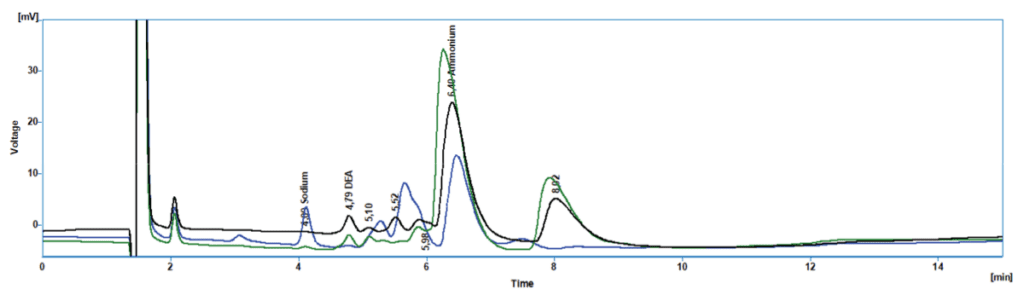
- Contaminants: Determine if DEA Breakdown products are present such as Ammonium, MEA, or TEA.
Determine Key Breakdown Products:
Diethanolamine (DEA) is a critical component in boiler water treatment, but it can degrade under certain conditions. Understanding these factors is essential for effective boiler water management.
Primary Causes of DEA Breakdown
- High Temperature: Higher boiler water temperatures can accelerate DEA breakdown. As the temperature rises, DEA molecules become more energetic, increasing the likelihood of chemical reactions and decomposition.
- Oxygen Exposure: The presence of dissolved oxygen in boiler water can oxidize DEA, leading to its degradation. Oxygen can enter the boiler system through incoming feedwater or leaks.
- pH Levels: Extreme conditions, whether too acidic or too alkaline, can destabilize DEA and promote its breakdown.
- Contaminants: The presence of certain impurities in the feed-water, such as heavy metals or organic matter, can catalyze the decomposition of Diethanolamine.
- Residence Time: Prolonged exposure of DEA to high temperatures and pressures can increase its breakdown rate.
Breakdown analytes to monitor:
- Ammonium: Ammonium is formed when DEA breaks down at higher temperatures. Ammonium can be monitored in the same analysis as the DEA on the IONUS IC.
- Organic Acids: Acetic acid and/or glycolic acid, in the water could harm the boiler.
- Anion Impurities: Chloride, Nitrite, Nitrate, and Sulfate are anionic components that can harm the boiler. Anions can be monitored on the IONUS IC as a separate analysis requiring additional hardware.
- Other Breakdown Amines: These can include Monoethanolamine (MEA), Triethanolamine (TEA), and other amine compounds that could be present when DEA breaks down, leading to increased pH levels that can increase scale formation or stress corrosion in the boiler. MEA and TEA can also contribute to foaming in the boiler, which can reduce heat transfer efficiency, causing water droplets to form in the steam system. These water droplets can be transported downstream, which could damage
turbines or other components. MEA and TEA can be monitored on the IONUS in the same analysis as the DEA on the IONUS IC.
Optimizing the Amine Regeneration Processes: Techniques such as electrodialysis are employed to remove impurities and regenerate spent Amine solutions. Analyzing the anion content before and after regeneration helps assess the effectiveness of the electrodialysis process and allows for adjustments to optimize its performance. This ensures the regenerated amine solution remains free of harmful anions for continued efficient CO2 capture.
Basic Principles of Ion Chromatography
IC is a sophisticated analytical tool that separates and identifies different ions (charged particles) in a solution. It’s like sorting different colored marbles based on their size and shape. In the case of boiler water, IC helps to identify the various chemicals, including DEA, and measure their concentrations.
The process involves:
- Sample preparation: A small amount of boiler water is taken and prepared for analysis.
- Injection: The prepared sample is injected into the IC system.
- Separation: The different ions in the sample are separated as they pass through a column.
- Detection: The separated ions are detected and measured.
- Data analysis: The IC system generates a report showing the concentration of each ion in the water.
The Benefits of IC Monitoring for DEA
Regular IC testing of boiler water offers several advantages:
- Early detection of problems: By identifying issues early on, you can prevent costly repairs or equipment failures.
- Optimized boiler performance:
Maintaining the correct chemical balance in the boiler water ensures maximum efficiency and energy savings.
- Extended boiler life: Protecting the boiler from scale and corrosion prolongs its lifespan.
- Reduced downtime: By preventing breakdowns, you minimize disruptions to your operations.
- Environmental protection: Proper boiler water treatment helps to reduce the environmental impact of your operations.
Bridging the Gap from Theory to Practice
Having established the critical role of DEA in refinery boiler water systems and the potential consequences of its breakdown, the transition from theoretical concepts to the practical application of monitoring and analyzing DEA levels is best accomplished with the Ion Chromatography analysis described below.
Accurate and timely DEA measurements are essential for maintaining optimal boiler performance, preventing equipment failures, and ensuring the overall efficiency of the refinery process.
Ion chromatography (IC) is a proven and preferred analytical technique for DEA determination due to its exceptional performance in handling complex matrices, ensuring high sensitivity, and offering remarkable selectivity. Its capabilities make IC an ideal choice for accurately measuring DEA levels in refinery boiler water systems.
The upcoming section, “The Analysis,” will share our proven, recommended approach for sample analysis with the IONUS IC to analyze DEA within this specific application. Our discussion will cover the fundamental principles of IC, including the chromatographic separation process, detection methods, and essential sample preparation techniques. We will explore how IC is uniquely suited to the analysis of DEA in refinery boiler water systems, taking into account factors such as potential matrix interferences, the analyte sensitivity, and the critical requirement for quick turnaround times.
Operating Conditions of the Method
| IC System | IONUS Ion Chromatograph |
| Column | Repromer Cat, 7 µm, 4 mm x 250 mm |
| Eluent | 3 mM HNO3 / 2.6 mM 18-Crown-6 |
| Flow Rate | 900 µL/min |
| Temperature | 40 °C |
| Injection | 20 µL |
| Detection | Conductivity, 1 mS/cm |
Standard Preparation
Certified Standards for Sodium (ICNA1) and Ammonium (ICNH41) were acquired from Inorganic Ventures (Christiansburg, VA). Each standard was provided with a Certificate of Analysis. The sodium certified value is 1002 ppm and the ammonium certified value is 1001 ppm.
Diethanolamine 99% (D83303-5G) was acquired from Sigma-Aldrich for use in preparing a 1000 ppm DEA stock standard. To prepare a 1000 ppm DEA stock standard from a 99% DEA solution equates to approximately 1 g/L. The formula below will provide the correct concentrations:
- Volume (mL) = (Mass of DEA needed / Desired concentration) * (100/Purity)
- Volume (mL) = (1 g DEA / 1g/L) * (100/99) = 1.01 mL
Using 1.01 mL of the DEA 99% with a total volume of 1000 mL produces a 1000 ppm DEA Stock Standard.
A working multi-ion standard was prepared from the certified Sodium, Ammonium, and the DEA 1000 ppm standard. This multi-ion standard was used in calibrating the IONUS IC.
| Analyte | Concentration |
| Sodium | 2.0 |
| DEA | 2.9 |
| Ammonium | 4.95 |
The instrument linearity for DEA was tested prior to analysis by preparing four levels of DEA in UHP DI water. Using the stock DEA standard, working standards of 1, 10, 20, and 50 ppm DEA can be prepared using this formula:
- 1000 ppm DEA x 0.1 mL / 100 mL UHP Di = 1 ppm DEA
- 1000 ppm DEA x 1.0 mL / 100 mL UHP Di = 10 ppm DEA
- 1000 ppm DEA x 2.0 mL / 100 mL UHP Di = 20 ppm DEA
- 1000 ppm DEA x 5.0 mL / 100 mL UHP Di = 50 ppm DEA
These working standards can be used to prepare your IC calibration curve or multi-ion mixed standards including sodium and ammonium are also options. Dilute samples as required to fit within the calibration curve for proper quantitation.
Sample Preparation
Boiler Water samples were received from the refinery and shaken vigorously to insure adequate mixing. An aliquot was removed from the sample and filtered with a 0.22um syringe filter.
Note: Boiler water may be different between locations and DEA levels may also differ between boilers. The DEA in our samples was filtered to prevent unseen particulates from contaminating our IC column. The samples were not diluted as they fit within our calibration curve. Samples were maintained at 20ºC to avoid any potential for bacteria growth.
The sample was loaded into the membraPure autosampler for analysis. The results were evaluated and calculated by the software Clarity (DataApex).

Conclusion
Monitoring DEA levels and other water quality parameters through IC is essential for maintaining the health and efficiency of your boiler system. It’s like getting regular check-ups for your boiler. By investing in regular IC testing, you’re safeguarding your investment, and ensuring optimal performance.
While the science behind IC might seem complex, understanding the importance of monitoring your boiler water is straightforward. By installing the IONUS Ion Chromatograph at your facility, the ease of use will simplify the acquisition of valuable data you need to make decisions about your boiler’s performance.
A well-maintained boiler is more efficient and reliable, saving money, and ensuring the boiler operations continue uninterrupted.
Results Table – Results in ppm
| Sample | Na | DEA | NH4 |
| 168-1 | 0.06 | 0.45 | 23.1 |
| 168-2 | Nd | 0.71 | 15.2 |
| 168-3 | 0.90 | 0.07 | 9.98 |
| 172-1 | 0.01 | 0.61 | 23.8 |
| 172-2 | 0.02 | 0.80 | 14.8 |
| 172-3 | 0.90 | 0.09 | 10.6 |
| QC | 1.99 | 2.89 | 4.96 |
| Std Values | 2.00 | 2.90 | 4.95 |
Chromatograms: