Quality control of proteins – basis for special medical food – by post-column derivatization with ninhydrin
Formula Food for special medical use, referred to as “special medical food”, is to meet the special needs of nutrition or diet for people with eating restriction, dyspepsia, metabolic disorder or specific disease state, a specially prepared formula.
These products are part of medical nutrition therapy and are regulated to ensure their safety, efficacy, and suitability for specific patient groups (IOM, 2011).
When the target population is unable to use ordinary diet or daily diet to meet their nutritional needs, special medical use formula food can play a nutritional support role as a nutritional supplement. In view of the specific metabolic state of different diseases, the formula food for special medical use has put forward special regulations on the corresponding nutrient content, which can better adapt to the specific disease state or the nutritional requirement of a certain stage of the disease, to provide targeted nutritional support for patients is an effective way for clinical nutritional support.
For example, specialized formulas are designed for patients with phenylketonuria, renal insufficiency, or inflammatory bowel disease, where altered protein and amino acid requirements are critical for disease management (Koletzko et al., 2015).
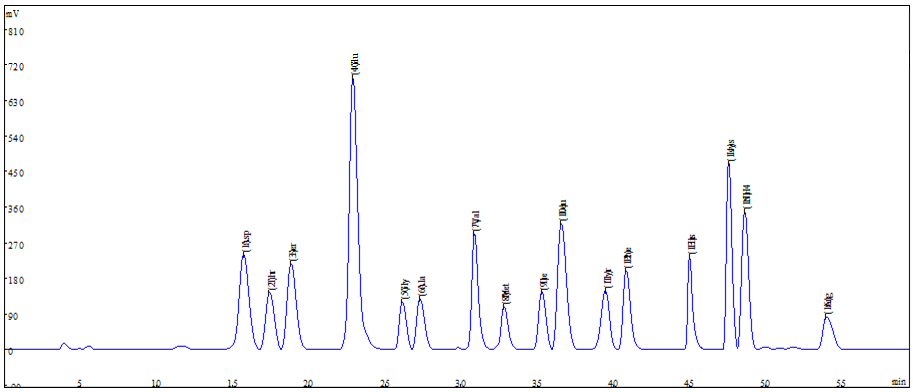
It is necessary to analyze the content and quality of protein and whether its amino acid composition meets the needs of human body. Amino acid analyzer is a special instrument for analyzing amino acid in national standard method.
Accurate amino acid profiling is crucial, as imbalances can negatively affect patient outcomes, particularly in populations with metabolic disorders or in critically ill patients (van Zanten et al., 2019).
In clinical nutrition, methods such as high-performance liquid chromatography (HPLC) combined with post-column derivatization are widely used to assess the amino acid composition in medical foods and ensure compliance with international nutritional standards (Rogers et al., 2020).
References:
- IOM (Institute of Medicine). “Dietary Reference Intakes: Applications in Dietary Planning.” Washington, DC: The National Academies Press, 2011.
- Koletzko B, et al. “Practical approaches to pediatric enteral nutrition: a consensus statement.” Nutrients. 2015;7(6):4817-4836.
- van Zanten AR, et al. “High-protein enteral nutrition enriched with immune-modulating nutrients vs standard high-protein enteral nutrition and nosocomial infections in ICU patients.” JAMA. 2019;321(24):2367-2379.
- Rogers S, et al. “Advances in post-column derivatization techniques for amino acid analysis.” J Chromatogr B Analyt Technol Biomed Life Sci. 2020;1155:122295.

